Bioethical Aspects of Translational Medicine
- Публикации /
-
1438
ABSTRACT
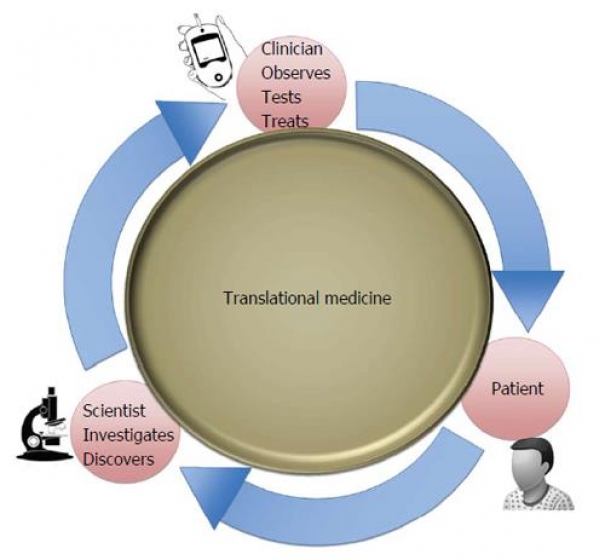
The development of modern medicine is associated with the dynamic translation (transfer) of basic medicine data to clinical research and further on to clinical practice. It is pointed out that research and development in the sphere of genetics and biotechnology, which are of particular significance during the COVID-19 pandemic, are of paramount importance in this regard. The concept of “translational distance” is analyzed as a measure of uncertainty, namely, the number and the size of logical leaps in course of transition from animal model trials to the first stages of human subject research. Translational research ethics has become a revolutionary, diverse, and distinct field of biomedical ethics. When studying the issue, special consideration is given to the critical blocks in translation as well as the characteristic features, types, and phases of translational research. It is emphasized that addressing the issue of minimizing irreducible uncertainty so that research participants could participate in research is a key component of ethical research. In view of the fact that the most important condition for the successful implementation of translational medicine is the adherence to the principles of bioethics when overcoming translational distances is analyzed taking into account the benefit-risk balance. As the development of translational medicine is significantly influenced by the legislation and the practice of its application, the national peculiarities of the attitude of different countries to the issues of ethics and the resolution thereof are studied, including the differences between the continental and the Anglo-Saxon legal families. Along with the formation of a general approach to the choice of a regulatory model in the sphere under consideration, the acceleration of circulation of the information related to science, research and technology, as well as the rapid obsolescence of innovations, should not be overlooked. At the same time, one should pay attention to the existing biological and other risks.
For citations:
Khokhlov A.L., Belousov D.Yu., Kagramanyan I.N., Mokhov A.A., Tsyzman L.G., Samarina E.I. Bioethical Aspects of Translational Medicine. Kutafin Law Review. 2022;9(1):175-190. https://doi.org/10.17803/2313-5395.2022.1.19.175-190
.png)